Molecular Motors in Action: Visualizing α-Cyclodextrin Movement Along Polymer Chains
Scientists visualize α-cyclodextrin rings moving along a polymer using fast-scanning atomic force microscopy, aiding molecular machine design
Polypseudorotaxanes, in which α-cyclodextrin (α-CD) rings shuttle along a poly(ethylene glycol) (PEG) chain, are promising candidates for molecular machines. However, their molecular dynamics have remained unclear. Researchers have now used fast-scanning atomic force microscopy (FS-AFM) to visualize α-CD rings moving along a PEG chain. This breakthrough establishes FS-AFM as a powerful tool for analyzing supramolecular polymers and paves the way for designing efficient molecular motors.
Imagine a microscopic locomotive moving back and forth along a track, propelling itself without any external force. At the molecular level, this concept forms the foundation of molecular motors-- intricate systems that could enable advanced materials, targeted drug delivery, and the development of nanoscale robotics.
Inspired by nature's molecular machines, scientists have been developing artificial counterparts since the first synthetic molecular machine was created in 1994. This research has progressed rapidly, culminating in the 2016 Nobel Prize in Chemistry for breakthroughs in molecular machine design. One promising candidate is polypseudorotaxane, a structure where a poly(ethylene glycol) (PEG) polymer chain is threaded through multiple α-cyclodextrin (α-CD) rings. In aqueous solutions, these rings self-assemble onto the PEG chain and move along its length. However, the specific structural changes behind this movement have remained unclear-- until now.
Recently, scientists from the Japan Advanced Institute of Science and Technology (JAIST) have visualized the dynamic shuttling of α-CD rings along the PEG chain in real time, revealing localized structural changes that were previously unclear. Using a specialized microscope called fast-scanning atomic force microscopy (FS-AFM), the team, led by Associate Professor Ken-ichi Shinohara, captured real-time images of α-CD rings moving along the PEG chain. Their study, published in Macromolecules on March 4, 2025, introduces a new method for analyzing the structure of supramolecular polymers-- an approach that was previously unattainable and could pave the way for more advanced molecular machines.
"Although PEG@α-CD polypseudorotaxane is widely used, the structural changes that occur as α-CD rings shuttle along the polymer chain remain poorly understood. By revealing its structure at the solid-liquid interface, our study will contribute to the development of synthetic polymer motors driven by thermal fluctuations," explains Dr. Shinohara.
To prepare the polypseudorotaxane, the researchers mixed PEG100k with α-CD in an aqueous solution and allowed the sample to rest for more than six hours. This process led to the formation of a white solid, which they then analyzed using FS-AFM in a 15 millimolar potassium chloride aqueous solution. Unlike regular optical microscopes, AFM uses an ultra-sharp tip on a tiny lever to scan surfaces, capturing nanoscale features and generating high-resolution images.
Imaging of the PEG100k chain alone revealed a highly flexible, dumbbell-shaped structure with globules at both ends. This flexibility gave it spring-like properties, allowing it to expand and contract freely. As a result, when relaxed, the chain appeared much shorter (averaging 48.1 nm) than its actual stretched-out length of 790 nm. When α-CD rings were added, they reduced the chain's flexibility. Imaging the PEG100k@α-CD polypseudorotaxane showed a significantly longer (499.6 nm on average) and a more rigid structure, with the end-cap formations preventing the α-CD rings from slipping off. Interestingly, despite being less flexible, the chain still exhibited a spring-like motion, as α-CD rings continued to shuttle along its length.
"We observed that the polypseudorotaxane exhibited shrinking and extending motions driven by the shuttling of α-CD rings along the polymer chain. These movements mainly occurred in the exposed, self-shrinking PEG segments, where repeated expansion and contraction were observed as the α-CD rings moved," explains Dr. Shinohara. Molecular dynamics simulations further confirmed these findings, reproducing the shrinking and extending motions observed in the FS-AFM experiments.
Although fully functional molecular machines remain a long-term goal, this study lays the groundwork for understanding molecular motion in supramolecular systems. "FS-AFM is a promising technique for analyzing supramolecular materials, especially when conventional spectroscopic methods are unsuitable for structural analysis," remarks Dr. Shinohara. These insights could lead to energy-efficient molecular motors that harness thermal energy at room temperature for controlled movement.
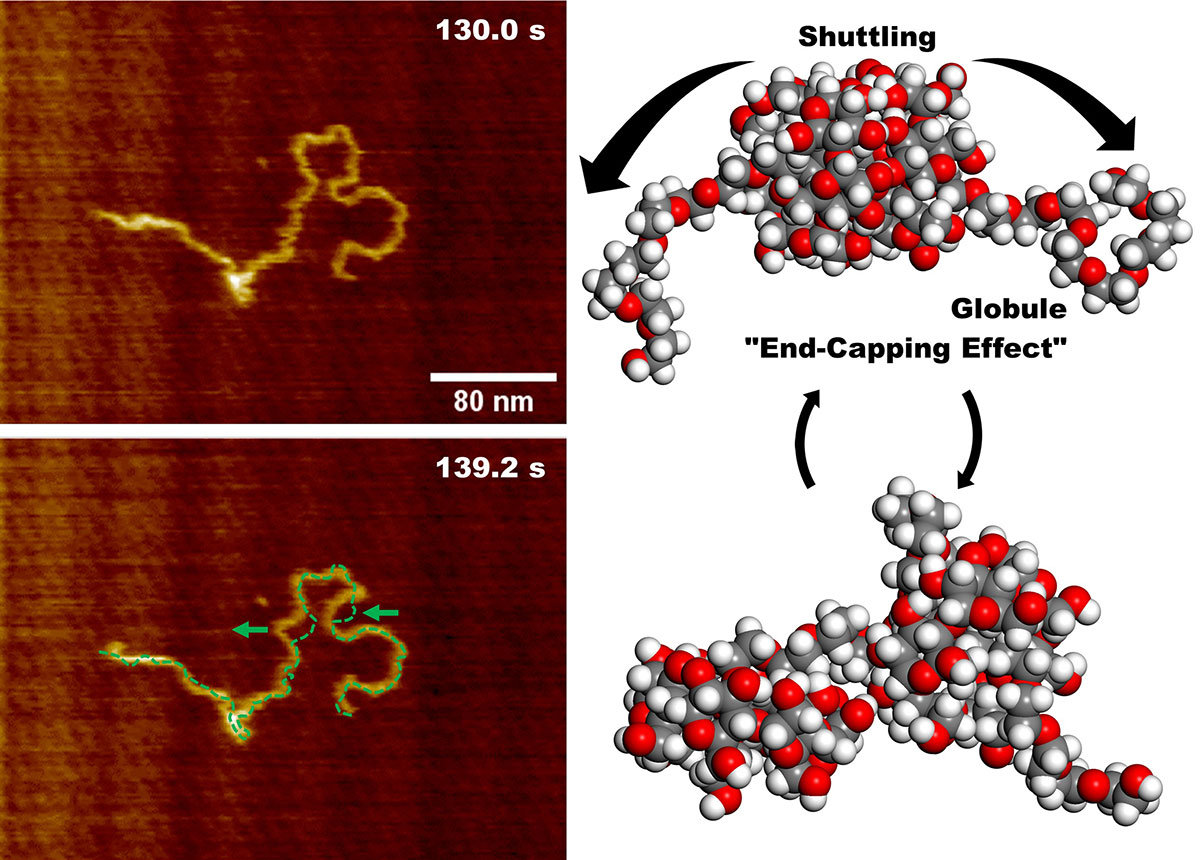
Image Title: Molecular motion of PEG100k@α-CD polypseudorotaxane
Image Caption: Fast-scanning atomic force microscopy imaging of the molecule at two different time points shows positional shifts along the polymer chain on the left. On the right, the molecular structure of PEG100k@α-CD polypseudorotaxane, illustrating the shuttling of α-cyclodextrin (α-CD) rings and the end-cap structures that prevent their release.
Image Credit: Reproduced from Hori and Shinohara with permission from Macromolecules, an ACS Publications journal
Image Source Link: https://pubs.acs.org/doi/10.1021/acs.macromol.4c02491
License Type: CC BY-NC-ND
Usage Restrictions: Credit must be given to the creator. No derivatives or adaptations of the work are permitted.

Image Title: Molecular dynamics simulations of PEG 20-mer chain with two α-CD rings
Image Caption: The images show the self-shrinking and extending motions driven by the shuttling of α-cyclodextrin (α-CD) rings, replicating the observations from fast-scanning atomic force microscopy imaging.
Image Credit: Reproduced from Hori and Shinohara with permission from Macromolecules, an ACS Publications journal
Image Source Link: https://pubs.acs.org/doi/10.1021/acs.macromol.4c02491
License Type: CC BY-NC-ND
Usage Restrictions: Credit must be given to the creator. No derivatives or adaptations of the work are permitted.
Reference
| Title of original paper: | Direct Observation of the "End-Capping Effect" of a PEG@α-CD Polypseudorotaxane in Aqueous Media |
| Authors: | Ryoga Hori and Ken-ichi Shinohara |
| Journal: | Macromolecules |
| DOI: | 10.1021/acs.macromol.4c02491 |
Funding information
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI through a Grant-in-Aid for Scientific Research (B) (Grant No. 20H02546) and a Grant-in-Aid for Scientific Research (C) (23K04520) (K.S.) and JST SPRING (JPMJSP2102) (R.H.).
March 11, 2025
