New Bacteria-Based Therapy Shows Promise for Fighting Cancer
Researchers develop a novel method to enhance the cancer-killing properties of bacteria residing in tumors
Bacteria-based therapies represent an exciting breakthrough in cancer treatment, harnessing their remarkable ability to specifically target and attack tumors. To fully harness the potential of this approach, an efficient and safe method for producing natural anticancer bacteria is needed. Now, researchers from Japan have developed a novel scaffold-based method to culture antitumor bacteria. This method not only enhances the tumor-killing properties and safety of the bacteria but also presents a simple approach for large-scale cultivation.
Even as cancer remains a leading cause of death globally, bacteria-based cancer therapy presents an exciting and innovative treatment option. Owing to their ability to penetrate the rigid stromal barrier, bacteria can naturally target solid tumors and offer intratumoural penetration. However, before these bacteria can be used in medical treatments, several important factors need to be addressed. Bacteria intended for clinical trials must be weakened or "attenuated" to ensure their safe use in animals and humans. Additionally, a simple manufacturing procedure is needed to produce safe and effective anticancer bacteria. This calls for the development of an optimal culturing method for bacteria.
Now, in a study published online in the Chemical Engineering Journal, researchers at the Japan Advanced Institute of Science and Technology (JAIST), led by Professor Eijiro Miyako and including Mikako Miyahara, in collaboration with researchers from the University of Tsukuba, Japan, have developed a new method to culture antitumor bacteria using highly porous scaffolds. This innovative approach not only enhanced the anticancer properties of the bacteria but also improved its safety in animal testing.
Previously, the research team isolated a group of bacteria, named A-gyo and UN-gyo from tumors in mice. A-gyo refers to the bacterium Proteus mirabilis and UN-gyo to the photosynthetic bacterium, Rhodopseudomonas palustris. These bacteria inhabit within tumor cells interacting with them, and potentially influencing tumor growth and response to treatment. Together, they form the 'AUN bacterial consortium', which shows great promise as a powerful tool for cancer detection because of its ability to effectively target tumors and its safety. However, finding the best way to grow these bacteria was challenging.
To address the challenges of culturing AUN, the researchers explored using specially designed scaffolds. They prepared the microporous scaffold using a biocompatible substance called polydimethylsiloxane (PDMS), combined with titanium dioxide (TiO2). The addition of TiO2 helped create a balance where the bacteria could effectively target tumors but were controlled enough to avoid overly aggressive bacterial growth leading to unintended infections or immune responses. These porous scaffolds significantly boosted the bacteria's anticancer properties, making them more effective.
Once the researchers prepared the PDMS-TiO2 composite, they cultured AUN bacteria along with blocks of the scaffold while exposing the entire setup to light. They found that when exposed to light, TiO2 in the scaffold effectively attenuated the bacteria by producing toxic molecules called reactive oxygen species (ROS), helping ensure safety during treatment.
Next, they evaluated the anticancer efficacy of AUN. Surprisingly, the researchers found that AUN cultured with the scaffold exhibited an enhanced ability to kill different types of tumor cells. When tested on mice with breast cancer, treatment with the attenuated AUN bacteria led to improved survival rates. Heightened anticancer activity was observed in mice with drug-resistant breast cancer.
"We discovered that the strong anticancer response was due to the oncolytic (cancer-killing) properties of AUN itself, with the assistance of various activated immune cells such as T cells, NK cells, and macrophages in the tumor microenvironment," says Mikako Miyahara, a doctoral student at JAIST and the lead author on the study. The study also confirmed that the AUN cultured with scaffolds could be safely administered not only to mice, but also to dogs.
The improved safety and efficacy of this simple method brings AUN one step closer to its widespread use in cancer treatment, and the authors of the study expect that this technology will be available for clinical trials within the next 10 years.
"Our discovery of how porous scaffolds influence the bacterial activities of AUN will help in designing artificial scaffold material for effective treatment against drug-resistant cancers," explains Prof. Miyako. In summary, this landmark study lays the groundwork for future commercialization and clinical use of AUN, bringing new hope to patients affected by various types of cancer.
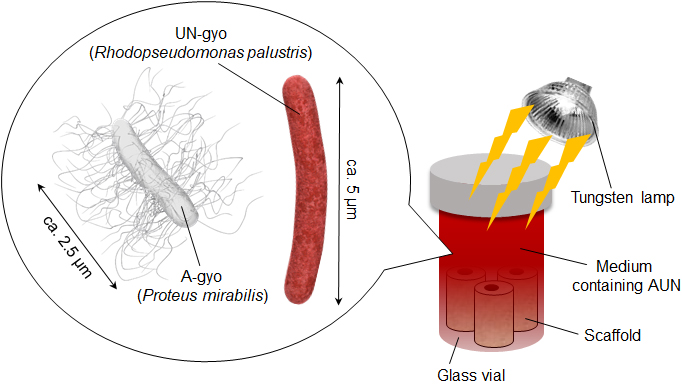
Image Title: Antitumor efficacy of AUN increased using scaffolds
Image Caption: An illustration showing the anticancer bacterial strains, A-gyo and UN-gyo, along with a simple method for growing AUN using a light-prepared scaffold.
Image Credit: Eijiro Miyako from JAIST
License Type: Original Content
Usage Restrictions: Cannot be used without permission.
Reference
| Title of original paper: | Photocatalytic scaffolds enhance anticancer performances of bacterial consortium AUN |
| Authors: | Mikako Miyahara, Yuki Doi, Naoki Takaya, Eijiro Miyako* |
| Journal: | Chemical Engineering Journal |
| DOI: | 10.1016/j.cej.2024.156378 |
Funding information
This work was financially supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid for Scientific Research (A) (Grant number 23H00551), JSPS KAKENHI Grant-in-Aid for Challenging Research (Pioneering) (Grant number 22K18440), the Japan Science and Technology Agency for Adaptable and Seamless Technology Transfer Program through Target-driven R&D (Grant Number JPMJTR22U1), Institute for Fermentation, Osaka (IFO), and the Uehara Memorial Foundation. M. M. thanks to JST SPRING (Grant number JPMJSP2102).
October 15, 2024
