
Dynamics of artificial cell membranes
Laboratory on Biological and Soft Matter Physics
Associate Professor:HAMADA Tsutomu
E-mail:
[Research areas]
Soft matter, Membrane biophysics
[Keywords]
Soft matter, Artificial cell, Biological membrane, Liposome, Phase separation, Molecular robotics
Skills and background we are looking for in prospective students
Basic skills of biochemical experiments, and interest in artificial cell membranes.
What you can expect to learn in this laboratory
- Experimental skills of artificial cell membranes
- Physical chemistry of soft matter
- Operation of optical microscopes
- Reading and understanding of scientific journals
【Job category of graduates】 Post-doc, Chemicals, Machinery
Research outline
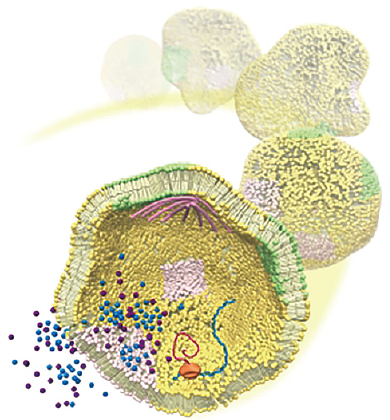 We use a soft matter physics approach to cell-mimicking systems so that we can understand the physical principles of biological systems. Living cells are a form of self-assembled soft matter. Lipid bilayer membranes are essential components of living organisms. We i) construct artificial lipid vesicles which produce cellular dynamics, such as endocytosis and autophagy, ii) elucidate the nonequilibrium membrane dynamics, and iii) conduct quantitative analyses based on condensed soft matter physics.
We use a soft matter physics approach to cell-mimicking systems so that we can understand the physical principles of biological systems. Living cells are a form of self-assembled soft matter. Lipid bilayer membranes are essential components of living organisms. We i) construct artificial lipid vesicles which produce cellular dynamics, such as endocytosis and autophagy, ii) elucidate the nonequilibrium membrane dynamics, and iii) conduct quantitative analyses based on condensed soft matter physics.
1. Membrane manipulation
We develop a novel system for photocontrol of dynamics of lipid vesicles through the use of a photosensitive molecule. We achieved membrane dynamics: i) triggering of lateral phase segregation, ii) budding vesicular transformation, iii) pore opening through the stabilization of bilayer edge, and iv) membrane fusion. The study could lead to a better understanding of the mechanism of membrane dynamics in living cells and may also see wider applications, such as in drug delivery and biomimetic material design.
2. Mechanical response of the membrane
We study a systematic analysis of lateral phase separation under mechanical stresses, such as tension and shear flow. We applied osmotic pressure directed toward the outside of vesicles to induce membrane tension. Microscopic observations clarified the shifts in phase structures within bilayer membranes with change in tension and temperature. We found that membrane tension can induce phase separation in homogeneous membranes. The study may provide insight into the biophysics of bilayer phase organization under tension, which is an intrinsic mechanical property of membranes.
Key publications
- "Photo-induced fusion of lipid bilayer membranes" Y. Suzuki, et al., Langmuir, 33, 2671 (2017).
- "Size-dependent partitioning of nano/micro-particles mediated by membrane lateral heterogeneity" T. Hamada, et al., J. Am. Chem. Soc., 134, 13990 (2012).
- "Domain dynamics of phase-separated lipid membranes under shear flow" T. Hamada et al., Soft Matter, 18, 9069 (2022).
Equipment
Fluorescence microscope
Optical microscope
Teaching policy
We aim to find novel phenomena of lipid vesicles to explore new possibilities of the membrane. Through research activities, we would like you to acquire skills with which you can solve a problem by using basic knowledge, and to experience pleasure of learning a thing with curiosity.
[Website] URL:https://www.jaist.ac.jp/ms/labs/hamada/english.html